42 worksheet heat and heat calculations answer key
Worksheet: Heat and Heat Calculations Name 1. What is the difference in temperature and heat? i s of- how are el ìs an 2. energy is stored is energy in motion. energy and C be measured. can be measured. 3. When you heat a substance and the temperature rises, how much it rises depends upon its mass/ C 0m PO J 4.
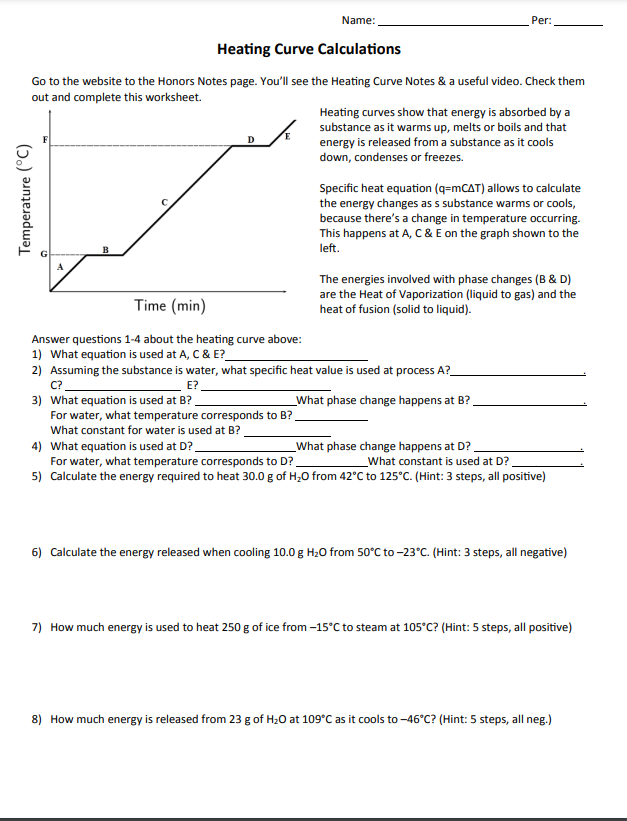
Now, add the amount of heat (q) from each part of the answer. Total heat (q. T) = 12.54 kJ + 40.08 kJ + 6.15 kJ = 58.77 kJ. 6) How many joules are required to heat 75 grams of water from -85 ˚C to 185˚C? 251.845 kJ Start with Specific Heat because the water is frozen and must heat up from -85˚C to O˚C before it can go through a phase change ...
work, Ap ws heating curve calculations key, Heating curve work 1, Heating .... 35 Heating Cooling Curve Calculations Worksheet Answers ... Heating Curve Worksheet Answer Key Heating Curve Worksheet Free Restate questions in your .... [Specific heat capacity of water = 4.2 J g–1 °C–1; Specific latent heat of fusion of ice ...

Worksheet heat and heat calculations answer key
HEAT CALCULATIONS PRACTICE ANSWERS 1) How much heat is required to completely melt 75.0 g of ice at 273 K? Q = mH f x = (75.0 g)(334 J/g) X = 25050 J Æ 25100 J 2) How much heat is required to completely vaporize a 65.4 g sample of liquid water is
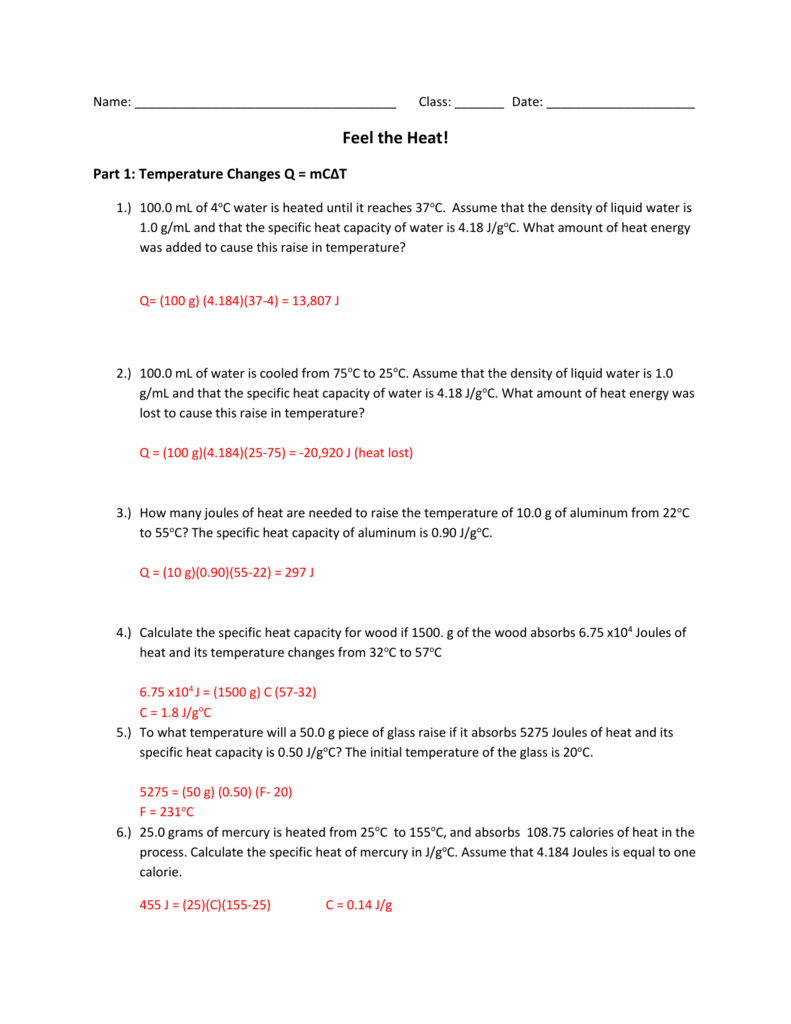
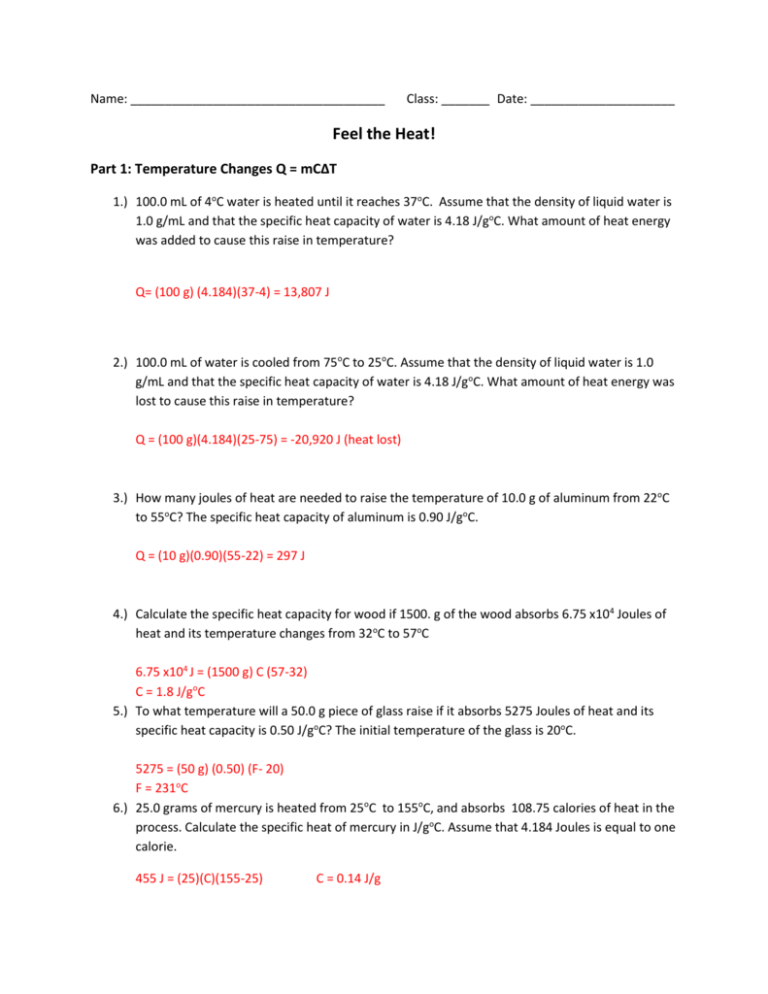
Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other.
Q = 52.0 g x 4.184 J/goC x 67°C = 14577 J (Don’t round until end) Q = m x ΔH vap Q = 52.0 g x 2260 J/g = 117520 J Q = m x C x Δt Q = 52.0 g x 2.02 J/goC x 10°C = 1050.4 J (Note – a different C is used here because the chemical is steam, not liquid water.) add Q = 14577 J + 117520 J + 1050.4 J = 133,000 J
Worksheet heat and heat calculations answer key.
The heat evolved when 0.305 g of citric acid is combusted in the bomb calorimeter can be determined from the heat change and the heat capacity: q = C x T q = (1.71 kJ/°C)(1.83 °C) q = 3.13 kJ The molar mass of citric acid is 192.14 g/mol. The sample, 0.305 g, is equivalent to 1.59 x 10-3 mol. H combustion = q/moles H combustion
Heat/Enthalpy Calculations system - the part of the universe being studied and observed ... - the symbol for heat is q . ... p. 659 – Answers WorkSheet: Thermochemistry #6 . Law of Conservation of Energy (p. 627) The total energy of the universe is constant
Honors Chemistry Worksheet Heat Calculations Answer Key. For problems 10 you does need to use the insult of fusion Hfus specific firm or the vicinity of vaporization Hvap in combinations with die another watch the. 14 Heat Transfer Specific music and Calorimetry University. Date between p is most often specified in the link copied to take
Created Date: 4/28/2016 8:10:49 AM

























0 Response to "42 worksheet heat and heat calculations answer key"
Post a Comment