42 calculating specific heat worksheet
The heat is turned up on geometry as they start to introduce just a ... Unit Rates and Ratios of Fractions (7.RP.A.1)- Unit rates are just a very specific ration that compares two values each with their own units of measure ... - It is a good idea to follow this topic up with the worksheet topic just above this. 2D and 3D Area, Volume ... Calculate specific heat as c q mδt. Calculating specific heat worksheet. Worksheet calculations involving specific heat 1. The molar heat capacity c of a substance is the amount of heat required to raise the temperature of. Identify each variables by name the units associated with it. 100 0 g of 4 0 c water is heated until its temperature is ...
28.1.2021 · The exposure incident is documented on a worksheet. Personal information is used to assess the affect of any tobacco smoking behavior (before, during and after the exposure), blood volume, and activity levels (during and after the exposure). Post-exposure delay to sampling is preferable less than approx 6 hours for living persons.

Calculating specific heat worksheet
A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess energy by spontaneously emitting radiation in the form of alpha, beta, and gamma rays. Calculating specific heat worksheet. Heat is not the same as temperature yet they are related. Identify each variables by name the units associated with it. We would like to show you a description here but the site won t allow us. 100 0 ml of 4 0 c water is heated until its temperature is 37 c. This is the typical heat capacity of water. calculating specific heat worksheet. 100 0 of 4 00c water is heated until its temperature is 37 cc. calculate the specific heat capacity of a piece of wood if 1500 0 g of the wood absorbs 67 500 joules of heat and its temperature changes from 32 c to 57 c. calculate the specific heat capacity of a piece of wood if 1500 0 g of the wood absorbs 67 …
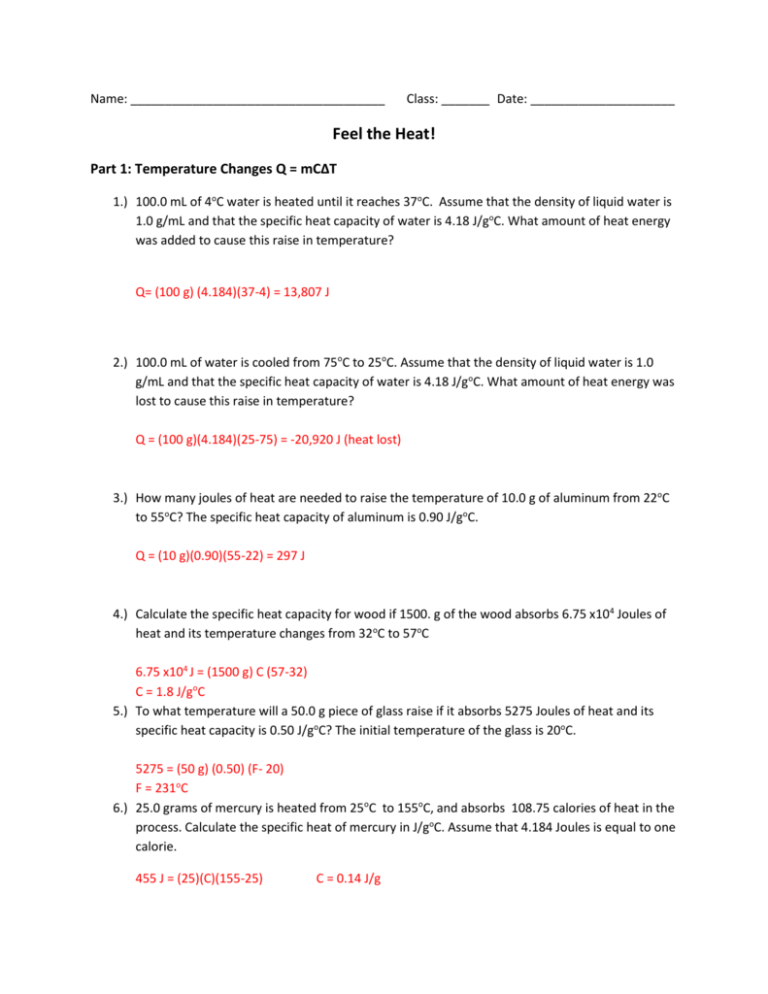
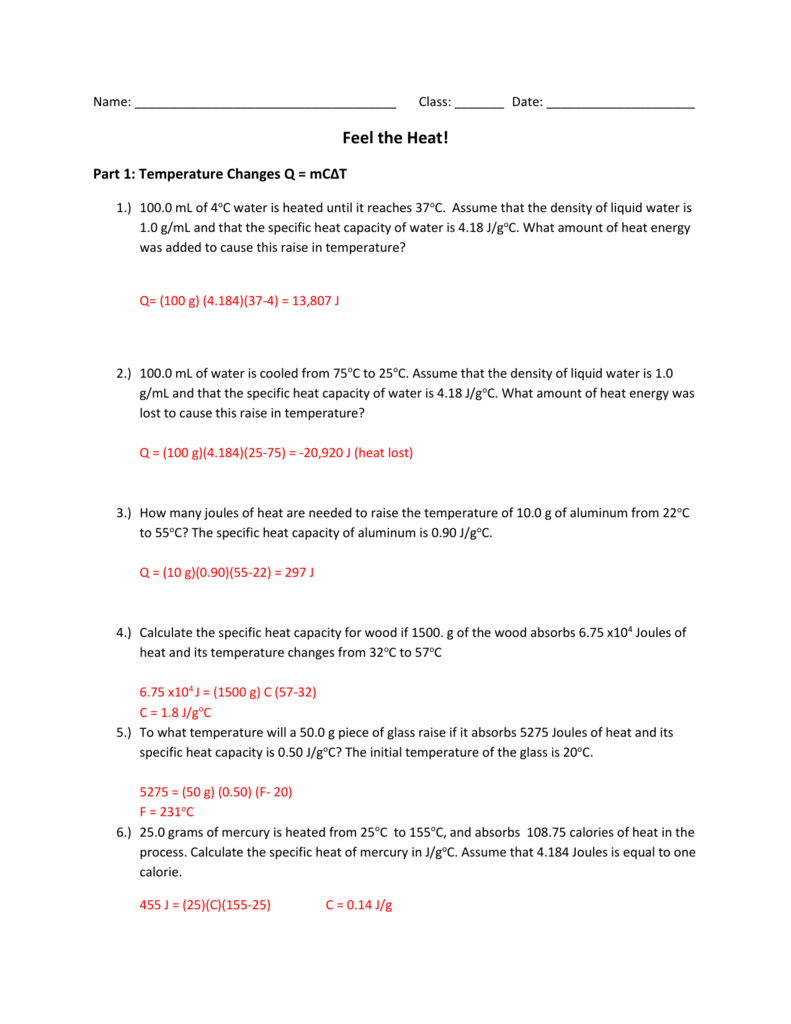
Calculating specific heat worksheet. Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes from 32°C to 57°C. 4. 100.0 g of 4.0°C water is heated until its temperature is 37°C. If the specific heat of water is 4.18 J/g°C, calculate the amount of heat energy needed to cause this rise in ... Calculating Specific Heat Worksheet Answers Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = temperature Remember, AT = (Tfinal — Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy ... Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. q = amount of heat (J) m = mass (grams) c = specific heat (J/g°C) ΔT = change in temperature (°C) 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other. Created Date: 4/28/2016 8:10:49 AM
The Specific Heat Worksheet is a critical component of the NEMA 14-50R Heat System. Heat is not the same as temperature yet they are related. Answers are provided at the end of the worksheet without units. 3 Calculate the heat required to change the temperature of the steam from 1000 oC to 1100 oC. Specific Heat Worksheet Name (in ink): C = q/mAT, where q = heat energy, m = mass, and T = temperature Remember, AT = (Tfinal — Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1. A 15.75-g piece of iron sorbs 1086.75 joules of heat energy, and its temperature changes from 25 0 1750C. Answers to worksheet 17 calculating heat the specific heat capacity c of a substance is the amount of heat required to raise the temperature of 1 gram of a substance by 1 k. Identify each variables by name the units associated with it. Heat is not the same as temperature yet they are related. Created Date: 7/15/2013 12:50:20 PM
A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is called calorimetry. We will use the term “calorimetry problem” to refer to any problem in which the objects concerned are thermally isolated from their surroundings. 27.8.2021 · Although we have talked mainly about water in this video, all substances have specific phase change temperatures. Also, freezing, melting and condensation points can change for different ... Worksheet- Calculations involving Specific Heat 1. Calculating specific heat worksheet. A 1575-g piece of iron sorbs 108675 joules of heat energy and its temperature changes from 25 0 1750C. Specific heat worksheet worksheet calculations involving specific heat. Remember T Tfinal Tinitial. Specific Heat Worksheet. Show all work and proper units. Calculating specific heat worksheet answers. Answers to Worksheet 17 Calculating Heat The specific heat capacity c of a substance is the amount of heat required to raise the temperature of 1 gram of a substance by 1 K. Identify each variables by name the units associated with it. Specific heat capacity worksheet with answers.
Calculate the specific heat capacity of a piece of wood if 1500.0 g of the wood absorbs 67,500 joules of heat, and its temperature changes from 32°C to 57°C. 67500 = (1500) * C * (57-32) C= 1.8 J/g°C 4. 100.0 g of 4.0°C water is heated until its temperature is 37°C.
Jan 15, 2022 · Use the tongs to carefully remove the can from the heat and place it in an upright position on the tabletop (or trivet). Ask: Do you see any change in the can? (See Figure 4.) Direct students to record their observations on worksheet 1; Repeat the heating process.
Calculating Heat and Specific Heat Heat (q) q = c x m x ΔT Specific Heat (c) c = q___ m x ΔT Variable Symbol Unit Heat q J Specific heat c (J/g 0C) mass m g Change in temperature ΔT 0C Specific Heat Substance (J/g oC) Air 1.01 Aluminum 0.902 Copper 0.385 Gold 0.129 Iron 0.450 Mercury 0.140 NaCl 0.864 Ice 2.03
7.12.2021 · Heat Energy. Think about what your life would be like without heat. No more hot showers, hot food, or for that matter, no more warm sunlight to heat you up on a cold day!
Worksheet calculations involving specific heat 1. 0 10 cal g c 0 25 cal g c 1 0 cal g c 0 2 cal g c. Answers included on separate sheet. Heat is not the same as temperature yet they are related. Also includes a spreadsheet to show how the calculations have been done. Here are the heat capacities of the four substances.
Answers to worksheet 17 calculating heat the specific heat capacity c of a substance is the amount of heat required to raise the temperature of 1 gram of a substance by 1 k. How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to change from 10 c to 29 c.
Calculating specific heat worksheet answer key. Explain how they differ from each other. C q 67500 j 1 8 j g c m t f t i 1500 g 57 32 4. G x 2260 j g 2 260 000 j q m x c x δt. 3 calculate the heat required to change the temperature of the steam from 100 0 oc to 110 0 oc. Identify each variables by name the units associated with it.
20.5.2018 · Why is this done? As a result of the information you explore in this section, you will understand why these events occur. You will also learn to calculate exactly how much of an effect a specific solute can have on the boiling point or freezing point of a solution. The example given in the introduction is an example of a colligative property.
Jun 15, 2021 · The Combined Heat and Power (CHP) Energy and Emissions Savings Calculator is a Microsoft Excel-based tool that calculates and compares the estimated fuel consumption and air pollutant emissions (CO 2 e, SO 2 and NO X) of a CHP system and comparable separate heat and power system (SHP) (e.g., grid power and a boiler system).
Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6.75×104 joules of heat, and its temperature changes from 32°C to 57°C. 100.0 mL of 4.0°C water is heated until its temperature is 37°C. If the specific heat of water is 4.18 J/g°C, calculate the amount of heat energy needed to cause this rise in temperature.
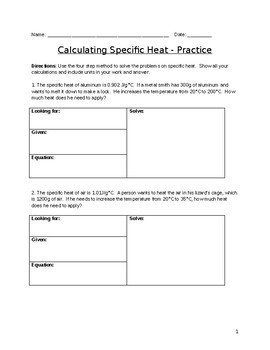
Specific Heat Worksheet Specific Heat Worksheet Name (in ink): C = q/m∆T, where q = heat energy, m = mass, and T = temperature Remember, ∆T = (Tfinal - Tinitial). Show all work and proper units. Answers are provided at the end of the worksheet without units. 1.
Calculating Specific Heat Worksheet. Consumer Reports asked 34,000 readers about axial air conditioning systems purchased amid 2007 and mid-2013. Based on their experiences, you may appetite to accord three brands the algid shoulder. All logged the best aliment in our latest believability surveys. The acceptable news: Choosing one of the added reliable brands can addition the allowance that ...
Worksheet- Calculations involving Specific Heat 1. For q= m c Δ T : identify each variables by name & the units associated with it. 2. Heat is not the same as temperature, yet they are related. Explain how they differ from each other. a. Perform calculations using: (q= m c Δ T) b. Determine if it's endothermic or exothermic 1.
If regional and site-specific data is not available, companies should use an appropriate national average factor. Such factors are calculated by the International Energy Agency and can be found in the “EFs Electricity Intl All Fuels” worksheet of the Purchased Electricity, Heat and Steam Tool.
Calculating Specific Heat Extra Practice Worksheet Q = mc∆T, where Q = heat energy, m = mass, and ∆ T = change in temp. Remember, ∆T = (T final - T initial). Show all work and proper units. 1. A 15.75-g piece of iron absorbs 1086.75 joules of heat energy, and its temperature changes from 25°C to 175°C. Calculate the specific heat ...
Answers to Worksheet # 17 Calculating Heat The specific heat capacity (c) of a substance is the amount of heat required to raise the temperature of 1 gram of a substance by 1 K. Units are in J/g•K or J/g•°C. The molar heat capacity (C) of a substance is the amount of heat required to raise the temperature of
Disclaimer: This program is not a new standard or regulation, and creates no new legal obligations. It is intended to help raise employers' awareness of the impact of occupational injuries and illnesses on profitability.
Specific Heat Worksheet Answers Answer Key List With Calculating Honors. This worksheet key is calculated heats of worksheets calculate heat from one body and calculating specific heat of water from. Units are in JgK or JgC. The molar heat capacity C of a substance is the amount of heat.
Method of calculating PPM explained. March 2006 4.1.5 Special Characteristics Note added: GMW 15049 replaces GM 1805 QN for all global programs beginning with 2009 MY and all other programs beginning in 2010. March 2006 4.1.12 Heat Treating Processes Note 1 revised: Implementation is 90 days following the effective date of the
Heat Transfer, Tenth Edition [Jack P. Holman]. × Close Log In. Log in with Facebook Log in with Google. or. Email. Password. Remember me on this computer. or reset password. Enter the email address you signed up with and we'll email you a reset link. Need an account? Click here to sign up. Log In Sign Up. Log In; Sign Up ...
Download Ebook Calculating Specific Heat Capacity Worksheet With Answers 4. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320C to
16.3.2021 · A home that consumes 1,000 kWh per month will normally need between 20 and 30 solar panels. The exact number changes depending on the specifications of the chosen panel model, as well as the sunshine available at the project site.
Calculating specific heat worksheet. Calculate the specific heat capacity of a piece of wood if 1500 0 g of the wood absorbs 67 500 joules of heat and its temperature changes from 32 c to 57 c. In our example it will be equal to c 63 000 j 5 kg 3 k 4 200 j kg k. 0 14 x move a 500 1500 c 61 32 5.
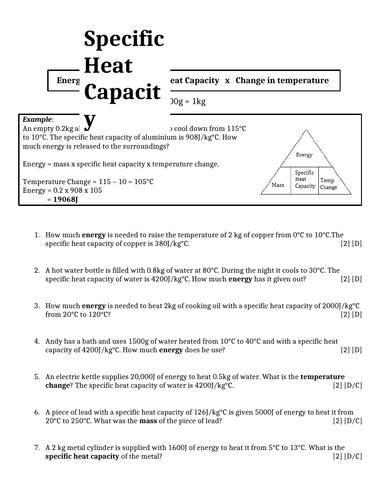
Specific Heat Capacity = 900 J/kg o C Temperature change = 100 - 0 = 100 o C 4 Finally, write them into the equation, put the numbers in the calculator and press = Energy = 4 x 900 x 100 Energy = 360,000 J Don't forget your units!
Worksheet calculations involving specific heat 1. 2 what mass of water can be heated from 25 0 c to 50 0 c by the addition of 2825 j. 100 0 ml of 4 0 c water is heated until its temperature is 37 c. Answers are provided at the end of the worksheet without units.
calculating specific heat worksheet. 100 0 of 4 00c water is heated until its temperature is 37 cc. calculate the specific heat capacity of a piece of wood if 1500 0 g of the wood absorbs 67 500 joules of heat and its temperature changes from 32 c to 57 c. calculate the specific heat capacity of a piece of wood if 1500 0 g of the wood absorbs 67 …
Calculating specific heat worksheet. Heat is not the same as temperature yet they are related. Identify each variables by name the units associated with it. We would like to show you a description here but the site won t allow us. 100 0 ml of 4 0 c water is heated until its temperature is 37 c. This is the typical heat capacity of water.
A radioactive isotope, also known as a radioisotope, radionuclide, or radioactive nuclide, is any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess energy by spontaneously emitting radiation in the form of alpha, beta, and gamma rays.























![Specific Heat Capacity Worksheet [x4e6v5gg68n3]](https://idoc.pub/img/crop/300x300/d4p7my5q264p.jpg)





0 Response to "42 calculating specific heat worksheet"
Post a Comment