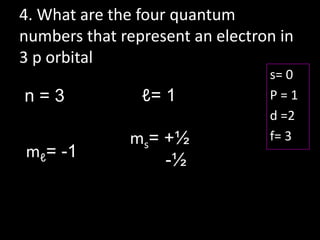
41 quantum number practice worksheet
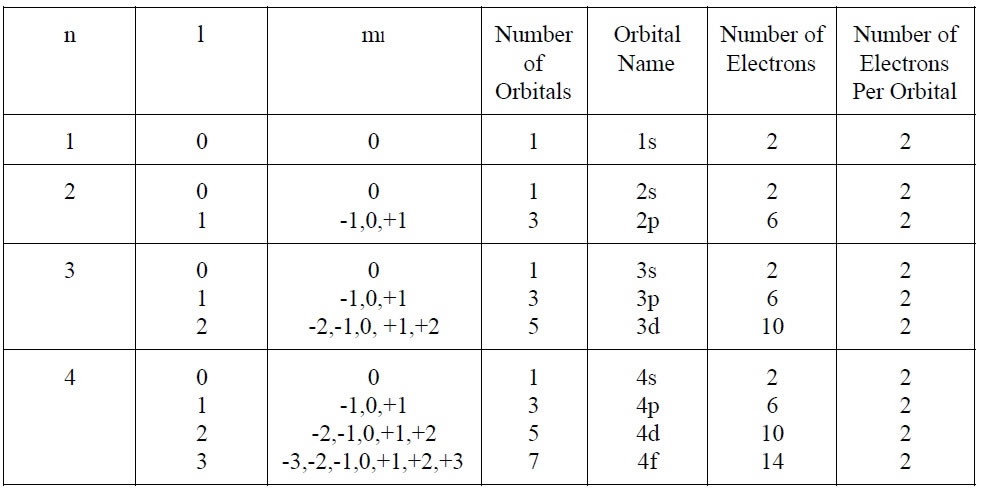
Quantum Numbers | Other Quiz - Quizizz For a principle quantum number, "n", equal to 6, is it possible for "l" to be equal to 7? answer choices yes no Question 9 120 seconds Q. The 4th energy level (n = 4) has how many total orbitals? answer choices 2 4 8 16 Question 10 120 seconds Q. Is the following quantum numbers (n, l, ml, ms) possible? (4, 1, -1, +1/2) answer choices yes no Quantum Number Practice Worksheet - Set 2 | PDF - Scribd summarize: 'the principal quantum number, m, can have the values of | 2 3 "i 5 ete, 'the angular momentum quantum number, can have integer values from) to.0 'the magnetic quantum number, m, can have integer values rom —£. to 72. 2, when n =3,teanhave values of__©2 /, 2 or the aonb thasa vate of, "on veanhave valuesor 2, when forth ap orbit …
DOC QUANTUM NUMBERS WORKSHEET - lakesidehs.dekalb.k12.ga.us QUANTUM NUMBERS WORKSHEET Name _____ 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum numbers a. n = 3, L = 0 b. n = 3, L = 1 c. n = 3, L = 2 d. n = 5, L = 0. 3. Give the n and L values for the following orbitals

Quantum number practice worksheet
ChemTeam: Quantum Number Problems Problems #1 - 10 Fifteen Examples Probs 11-25 Examples and Problems only (no solutions) Return to Electrons Menu Problem #1: Which of the following is a possible set of quantum numbers that describes an electron? Solution: The solution is to look at each one and see if there is a violation of the rules for each quantum number. Quantum Numbers Chemistry Worksheet - qstion.co Quantum numbers chemistry worksheet. N = 5, l = 0 3. Element 1s 2s 2p 3s 3p 4s 3d quantum numbers 1. Also download free pdf chemistry class 11 assignments and practice them daily to get better marks in tests and exams for grade 11. In this worksheet, we will practice using quantum numbers to describe an electron within an atom. PDF QUANTUM NUMBERS WORKSHEET answers - lcps.org QUANTUM NUMBERS WORKSHEET 1. State the four quantum numbers, then explain the possible values they may have and what they actually represent. n - Pricipal Quantum Number: represents the energy level the electron is in, linked to the periods of the periodic. Can be 1 to 7 l - Secondary Quantum Number/Orbital Shape Quantum number: represents ...
Quantum number practice worksheet. Quantum Numbers Teaching Resources | Teachers Pay Teachers Quantum Numbers Notes and Practice Worksheet by Kevin Wahlmark 4.7 (15) $1.00 Zip Quantum numbers can be difficult to teach. Use this graphic organizer to help structure your students' notes, and then check their understanding with the accompanying practice worksheet. Quantum Numbers Questions - Practice Questions of Quantum ... Quantum Numbers Questions Quantum numbers could be used to define the trajectory and the movement of an electron in an atom. When all of the electrons in an atom's quantum numbers are put together, the Schrodinger equation should be fulfilled. Quantum numbers determine the values of a quantum system's fixed quantities. Quantum Number Practice Questions - Chemistry MCQs - Examsegg Learning Quantum Numbers practice problems with answers: Quantum number, Hund's rule, Electronic configuration, Aufbau's principle, Pauli's exclusion principle and Shape of orbitals practice multiple choice questions. Ques. Following Hund's rule which element contains six unpaired electron (a) Fe (b) Co (c) Ni (d) Cr View Answer PDF Orbitals and Quantum Numbers Practice Questions - University of Texas ... Orbitals and Quantum Numbers Practice Questions 1. What are the shapes of s, p, and d orbitals respectively? ... Describe the electrons defined by the following quantum numbers: n l ml 3 0 0 3s electron or orbital 2 1 1 2p electron or orbital 4 2 -1 4d electron or orbital 3 3 2 not allowed (l must be < n)
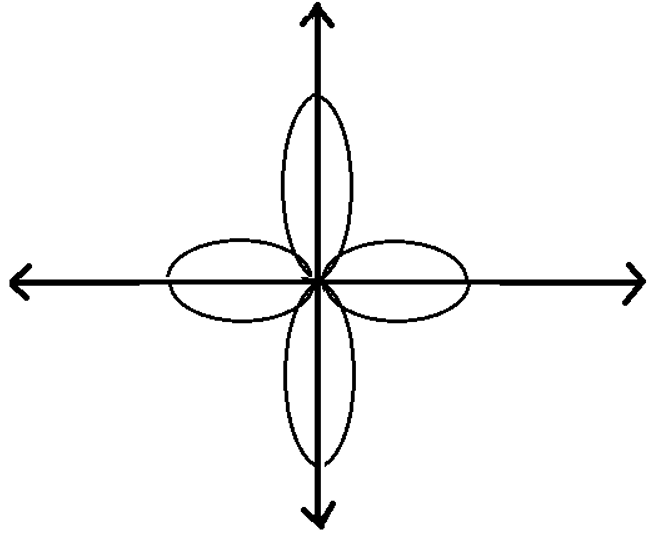
Quantum Numbers Worksheet copy.pdf - Name: _ Quantum Number Practice ... View Quantum Numbers Worksheet copy.pdf from CHEM 151 at Chandler-Gilbert Community College. Name: _ Quantum Number Practice Worksheet Date: _ 1. Summarize: The principal quantum number, n, can have DOCX QUANTUM NUMBERS WORKSHEET - hudson.k12.oh.us QUANTUM NUMBERS WORKSHEETName ________________________________ 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum number a. n = 3, l = 0 b. n = 3, l = 1 c. n = 3, l = 2 d. n = 5, = 0 3. Give the n and l values for the following orbitals Quantum Number Worksheet.pdf - Google Docs Quantum means that electrons exist in specific energy levels. This means that electrons must be in a particular energy level and can never be between energy levels. 2. Draw the shapes of the different types of orbitals: s p d 3. What are the four quantum numbers called? Principal, Angular Momentum, Magnetic and Spin Quantum number 4. Quantum Numbers Worksheets - K12 Workbook Displaying all worksheets related to - Quantum Numbers. Worksheets are Work quantum numbers, Quantum numbers work answers, Quantum numbers work, Name date quantum number practice work, Quantum numbers and electron configurations, Work quantum mechanics, Work 10, Quantum numbers and atomic orbitals. *Click on Open button to open and print to worksheet.
1.5.1- Quantum Numbers Worksheet KEY - StuDocu First - Primary Quantum number (n) = size of electron cloud n = 1 up to ∞... reality n = 1 - Second – Azimuthal or Angular Momentum Quantum number (( l ) = shape of electron cloud; l = 0 up to (n-1).... When l = 0 (s cloud), 1 (p cloud), 2 (d cloud), 3 (f cloud) Third – Magnetic Quantum number (m l ) = location or spatial orientation m l = - l to l Quiz & Worksheet - Electron Configurations & the Four Quantum Numbers ... Print Worksheet. 1. Which of the following is NOT true about the principal quantum number? It tells us the energy level of an electron. It tells us the size of an electron's orbital. It tells us ... PDF QUANTUM NUMBERS WORKSHEET - lee.k12.nc.us QUANTUM NUMBERS WORKSHEET Name _____ 1. State the four quantum numbers and the possible values they may have. 2. Name the orbitals described by the following quantum numbers a. n = 3, L = 0 b. n = 3, L = 1 c. n = 3, L = 2 d. n = 5, L = 0 3. Give the n and L values for the following orbitals a. 1s b. 3s c. 2p d. 4d e. 5f Quiz - Quantum Numbers Test Your Knowledge Now! 1. The magnetic quantum number describes the: number of electrons. average distance of the most electron-dense regions from the nucleus. spatial orientation of the orbital. shape of the orbital. 2. There are ___ types of quantum numbers 2 5 7 4 3. The principle quantum number is related to: the shape of the orbital.
Quantum Numbers (Worksheet) - Chemistry LibreTexts What is the maximum number of electrons in an atom that can have the following quantum numbers: n = 2, ‐ m s = ‐ 1 / 2 n = 5, l = 3 n = 4, l = 3, ‐ m l = ‐ 3 n = 4, l = 1, m l = 1 Q8. Write the condensed electron configurations for the following atoms, using the appropriate noble‐gas core abbreviations: C s N i S e C d A c P b Q9.
Quiz & Worksheet - Principal Quantum Number | Study.com The quiz and worksheet ask you to: Identify the value with the highest ionization energy Determine how many nodes are in a given value Recognize facts about quantum numbers Find the principle...
DOCX Quantum Numbers Practice Worksheet - Lincoln County R-III School District Quantum Numbers Practice Worksheet Quantum Numbers Practice WorksheetName: KEY Give the element and the electron configuration notation for the following: Quantum Number Element Identification Noble Gas Notation 2, 1, +1, +½ N [He] 2s 2 2p 3 3, 2, +1, -½ C u Ar 4s 2 3d 9 5, 3, 0, -½ Es [Rn] 7s 2 5f 11 7, 0, 0, -½ Ra [Rn] 7s 2
DOC QUANTUM NUMBERS WORKSHEET - Loudoun County Public Schools QUANTUM NUMBERS WORKSHEET 1. State the four quantum numbers, then explain the possible values they may have and what they actually represent. 2. State the number of possible electrons described by the following quantum numbers a. n = 3, l = 0 b. n = 3, l = 1 c. n = 3, l = 2, ml = -1 d. n = 5, l = 0, ml -2, ms -1/2 3.
Quantum Numbers Worksheets Teaching Resources | Teachers Pay Teachers Quantum Numbers Worksheet - On this practice worksheet, students determine the noble gas configurations, draw orbital diagrams, find the quantum numbers for the last electron and complete lewis structures. This worksheet is organized and formatted to help students understand the process of finding quantum numbers.
Quantum Number Problems Worksheets - K12 Workbook Quantum Number Problems Displaying all worksheets related to - Quantum Number Problems. Worksheets are Quantum numbers work answers, Orbitals and quantum numbers practice questions, Name date quantum number practice work, Quantum numbers work, Quantum numbers work, Quantum model work, , Quantum numbers work key.
PDF Name: Date: Quantum Number Practice Worksheet Quantum Number Practice Worksheet 8. (a) For n = 4, what are the possible values of l ? (b) For l = 3, what are the possible values of ml? 9. Give the values of n, l , ml (a) for each orbital in the 4f sublevel, (b) for each orbital in the n = 2 shell. 10. Which of the following sets of quantum numbers are allowed for an electron in an orbital ...
PDF Quantum Number Practice Worksheet Answers - howechem.net Quantum Number Practice Worksheet C) 1 9-3 8. (a) For n = 4, what are the possible values of I ? ... The quantum numbers listed below are for four different electrons in the same atom. Arrange them in order of increasing energy. Indicate whether any two have the same energy. (a) n = 4, 1=0, ml = O, ms = 1/2 ...
PDF Quantum Number Practice Worksheet - Weebly 12. What is the maximum number of electrons in an atom that can have the following quantum numbers: (a) n = 3 (b) n = 4, l = 2 (c) n = 4, l = 3, m l = 2 (d) n = 2, l = 1, m l = 0, m s = - ½ 13. The quantum numbers listed below are for four different electrons in the same atom. Arrange them in order of increasing energy.
Lesson Worksheet:Quantum Numbers | Nagwa Lesson Worksheet: Quantum Numbers Chemistry • Second Year of Secondary School. Lesson Worksheet: Quantum Numbers. In this worksheet, we will practice using quantum numbers to describe an electron within an atom. The quantum numbers for three electrons in an atom of silicon are shown.
2.2: Atomic Orbitals and Quantum Numbers (Problems) PROBLEM \(\PageIndex{2}\) Describe the properties of an electron associated with each of the following four quantum numbers: n, l, m l, and m s. Answer. n determines the general range for the value of energy and the probable distances that the electron can be from the nucleus.l determines the shape of the orbital.m l determines the orientation of the orbitals of the same l value with respect ...
PDF QUANTUM NUMBERS WORKSHEET answers - lcps.org QUANTUM NUMBERS WORKSHEET 1. State the four quantum numbers, then explain the possible values they may have and what they actually represent. n - Pricipal Quantum Number: represents the energy level the electron is in, linked to the periods of the periodic. Can be 1 to 7 l - Secondary Quantum Number/Orbital Shape Quantum number: represents ...
Quantum Numbers Chemistry Worksheet - qstion.co Quantum numbers chemistry worksheet. N = 5, l = 0 3. Element 1s 2s 2p 3s 3p 4s 3d quantum numbers 1. Also download free pdf chemistry class 11 assignments and practice them daily to get better marks in tests and exams for grade 11. In this worksheet, we will practice using quantum numbers to describe an electron within an atom.
ChemTeam: Quantum Number Problems Problems #1 - 10 Fifteen Examples Probs 11-25 Examples and Problems only (no solutions) Return to Electrons Menu Problem #1: Which of the following is a possible set of quantum numbers that describes an electron? Solution: The solution is to look at each one and see if there is a violation of the rules for each quantum number.





























0 Response to "41 quantum number practice worksheet"
Post a Comment