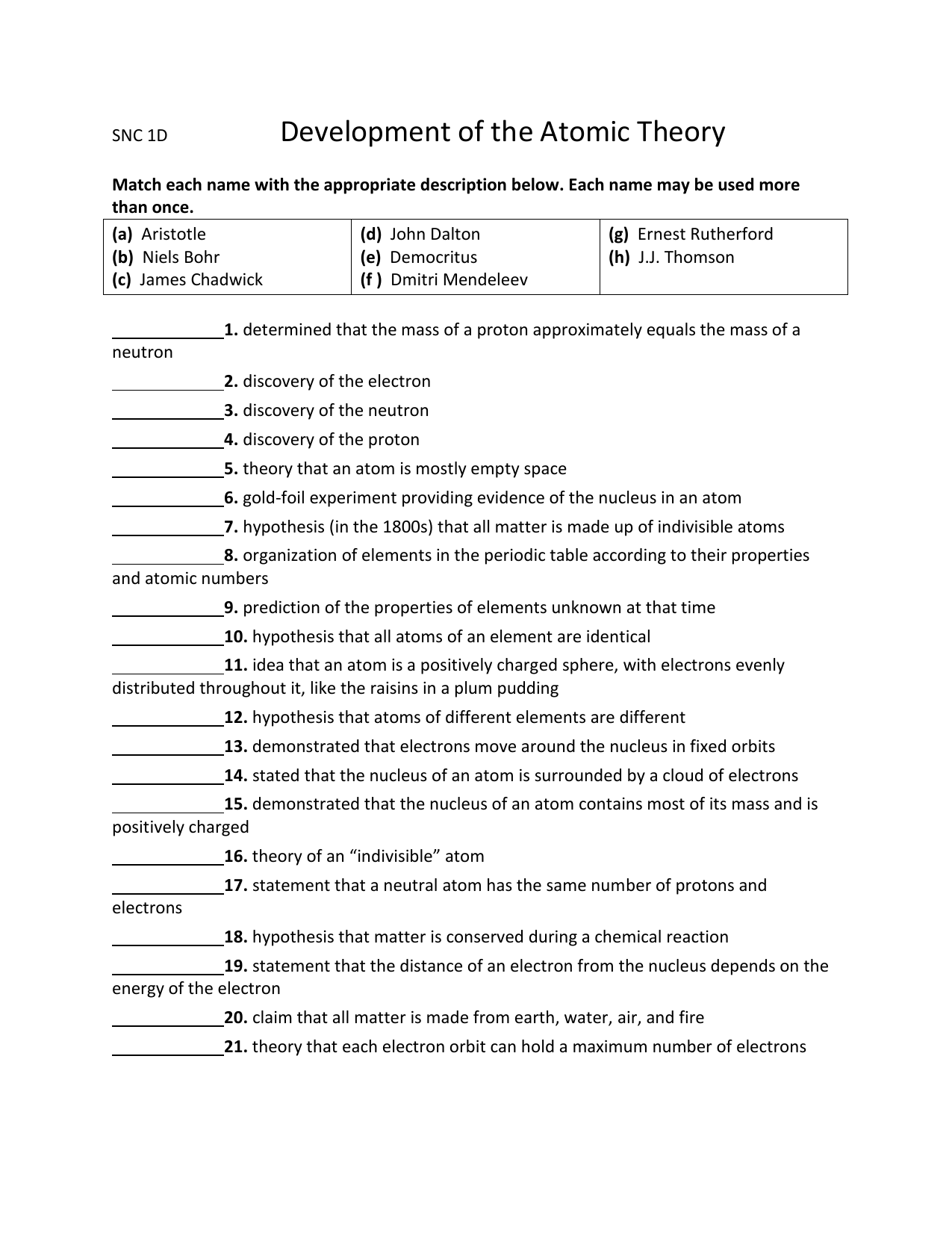
45 chemistry development of the atomic theory worksheet answers
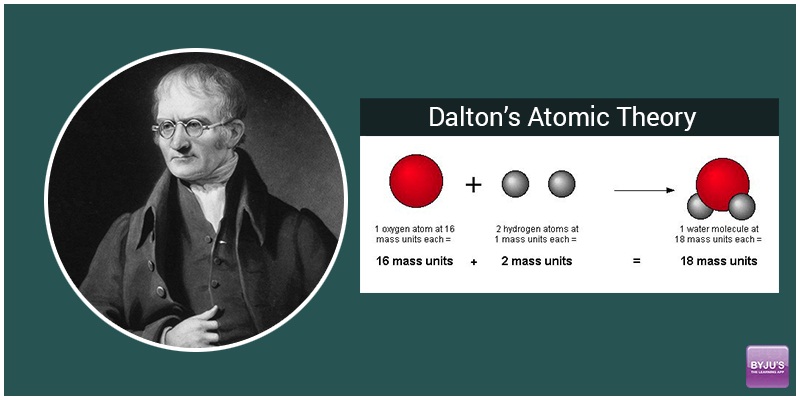
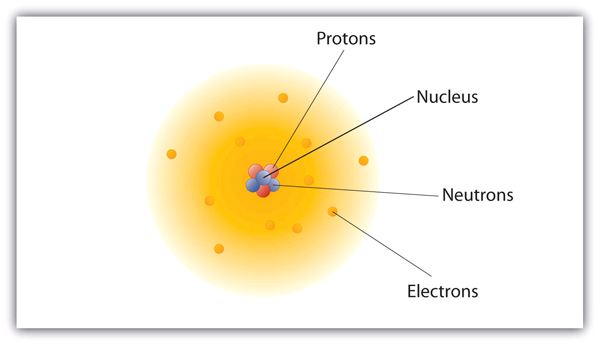
› atomic-structure-andAtomic Structure & The Changing Models of Atom - BioChem Tuition Sep 24, 2015 · Fig. 2. John Dalton used his own symbols to represent atoms and also developed the first table of atomic masses in New system of Chemical Philosophy (1808). John Dalton was the founder of the atomic theory. The main points of his atomic theory were: Elements are made up of tiny particles called atoms. Atomic number, atomic mass, and isotopes (article) - Khan Academy
study.com › skill › learnHow to Calculate the Mechanical Advantage of a Pulley - Study.com Step 1: Determine the number of movable pulleys, or the number of cord sections that support movable pulleys. By looking at the image, we can see that there is one moving pulley, the pulley on the ...

Chemistry development of the atomic theory worksheet answers
edu.rsc.org › practical › conservation-of-massConservation of mass | practical videos | 14–16 years Specification. England. GCSE. AQA Chemistry. 4.3 Quantitative chemistry. 4.3.1 Chemical measurements, conservation of mass and quantitative interpretation of chemical equations Atomic Models - WikiLectures List of the Atomic Theories in Order - Toppr
Chemistry development of the atomic theory worksheet answers. › indexPHSchool.com Retirement–Prentice Hall–Savvas Learning Company PHSchool.com was retired due to Adobe’s decision to stop supporting Flash in 2020. Please contact Savvas Learning Company for product support. › document › 524079710Cambridge IGCSE Chemistry Coursebook 4th Edition Atomic theory In a chemical reaction: Elements and compounds mix and react to produce new chemical substance(s) are formed the world around us. They produce massive objects usually the process is not easily reversed such as the ‘gas giants’ (the planets Jupiter and Saturn), energy is often given out. and tiny highly structured crystals of ... en.wikipedia.org › wiki › ChemistryChemistry - Wikipedia Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Sulfur | S (Element) - PubChem
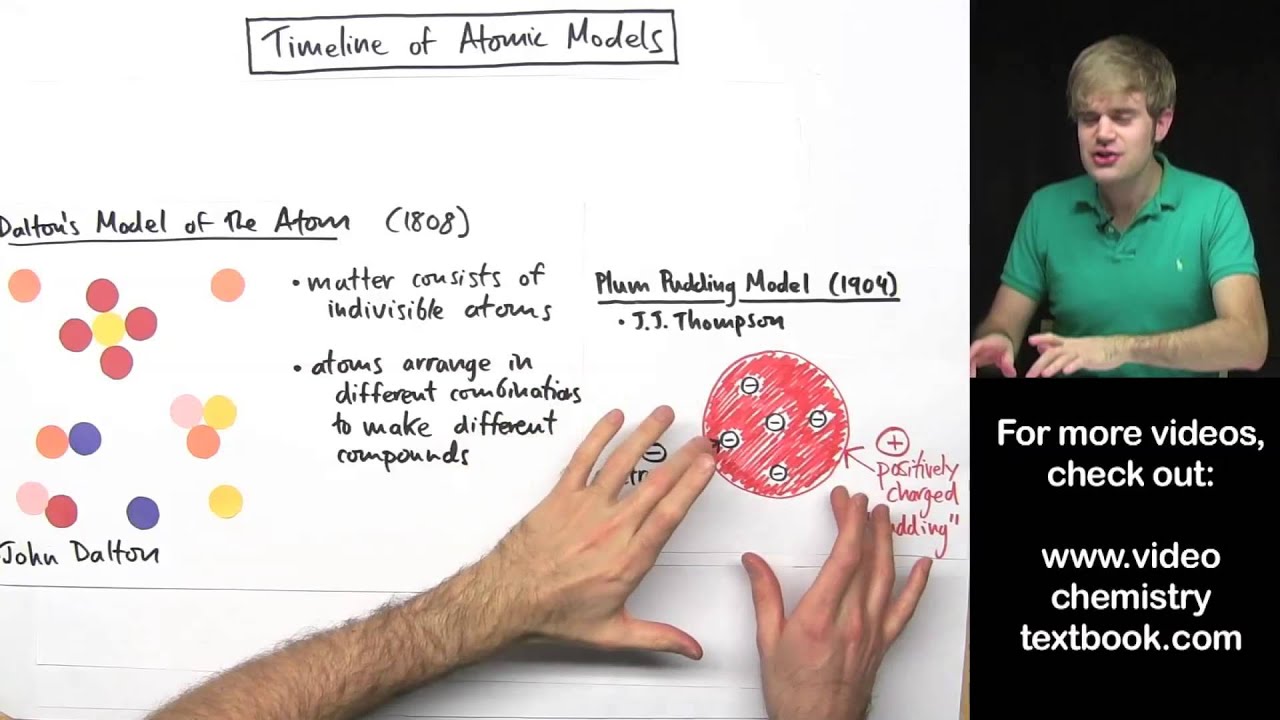
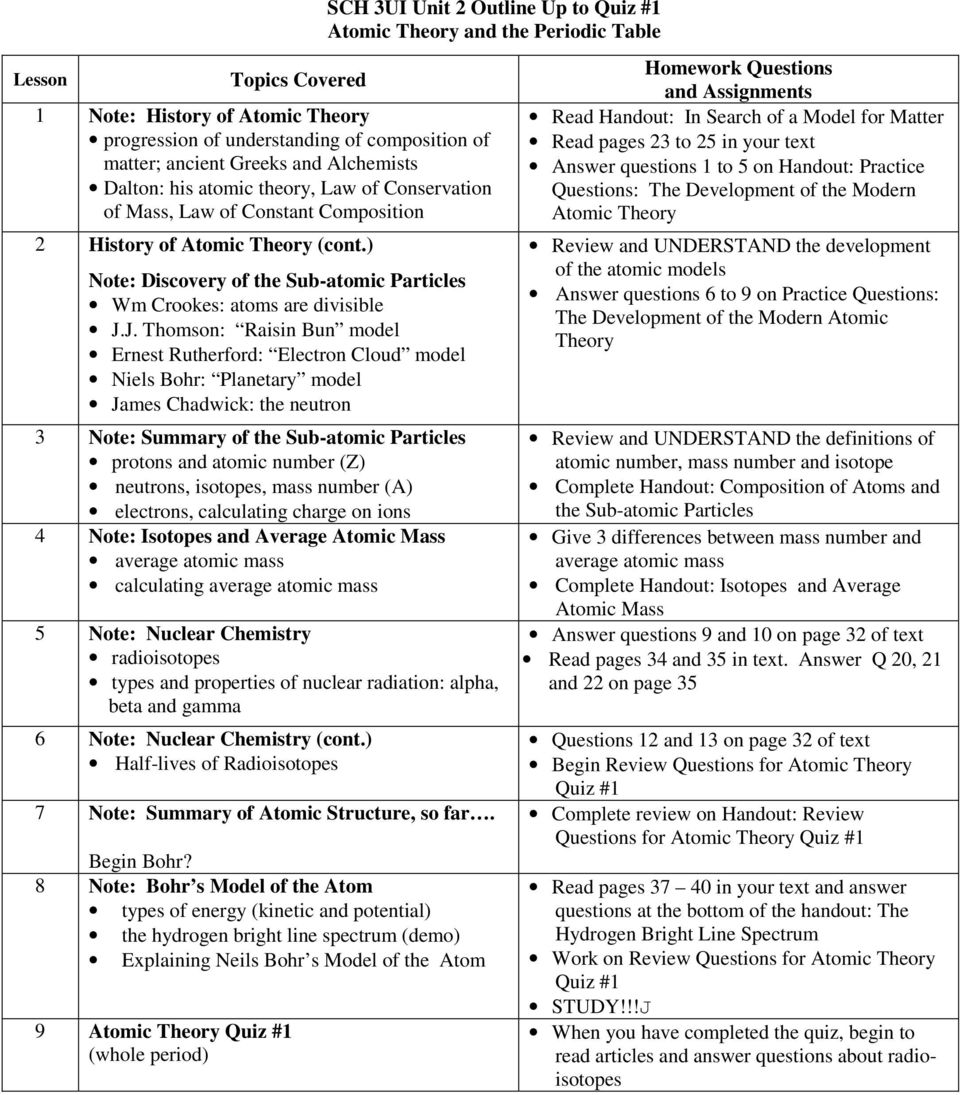
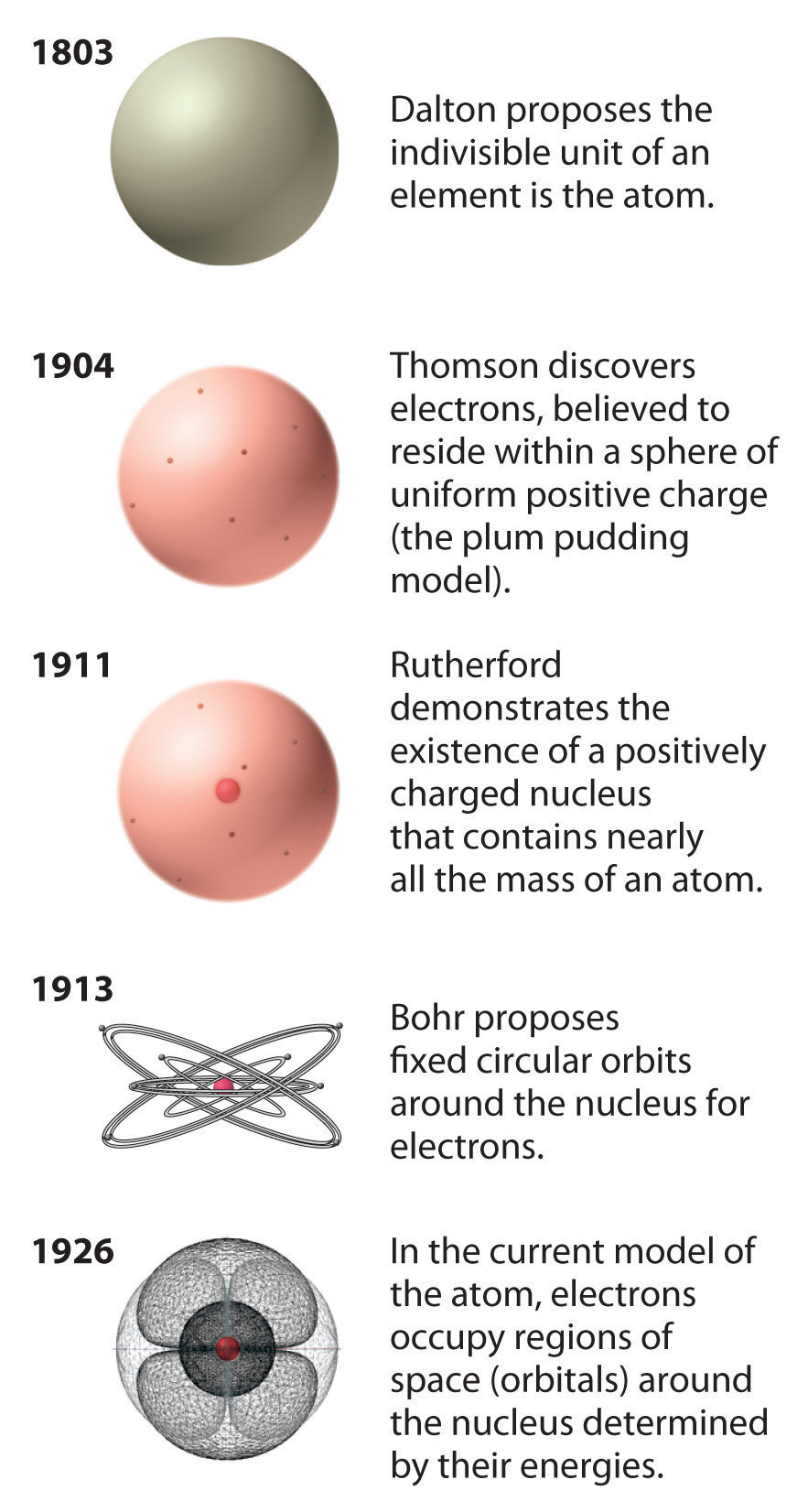
What Are The Different Atomic Models? Dalton, Rutherford, Bohr ... What Is An Atom ? | NRC.gov List of the Atomic Theories in Order - Toppr Atomic Models - WikiLectures
edu.rsc.org › practical › conservation-of-massConservation of mass | practical videos | 14–16 years Specification. England. GCSE. AQA Chemistry. 4.3 Quantitative chemistry. 4.3.1 Chemical measurements, conservation of mass and quantitative interpretation of chemical equations































0 Response to "45 chemistry development of the atomic theory worksheet answers"
Post a Comment